Our Technology
Measuring, predicting, and engineering protein-protein interactions
Our Experimental Platform
AlphaSeq
We use synthetic biology to generate high-resolution protein binding data at scale.
Vastly Multiplexed
Characterize whole protein-protein interaction networks with millions of interactions
Highly Quantitative
Measure physiologically relevant interaction strengths spanning picomolar to micromolar affinities
Widely Versatile
Antibody discovery, molecular glue target discovery, affinity tuning, protein interface engineering, and more
Our Computational Platform
AlphaBind
We apply machine learning (ML) to accelerate discoveries and engineer better therapeutics.
Predict Affinities
Learn from over 750 million affinity measurements to predict binding strength from protein sequence
Capture Diversity
Explore a massive sequence space around the parental by learning the effects of stacked mutations
Iterate Rapidly
Cycle from model-proposed sequences to binding data in six weeks to quickly validate and refine models
Our PROCESS
AlphaSeq + AlphaBind Explained
![]() AlphaSeq
AlphaSeqBUILD
Two yeast surface display libraries are built from synthesized or template DNA. Displayed proteins may include antibodies, cell surface receptors, intracellular proteins, bacterial or viral antigens, peptides, and more. The libraries are mixed in a liquid culture, allowing A and Alpha cells to collide. Small molecules can also be added to measure their effect on protein interactions — extending AlphaSeq into three dimensions![]()
FUSE
As cells collide, proteins on the cells’ surfaces may lead them to bind, where the probability of binding depends on protein interaction strength. Each surface expressed protein is paired with multiple unique DNA barcodes. Bound A and Alpha cells fuse, combining DNA barcodes into a single fused cell. A recombination event then pairs the barcodes onto one strand of DNA.![]()
MEASURE
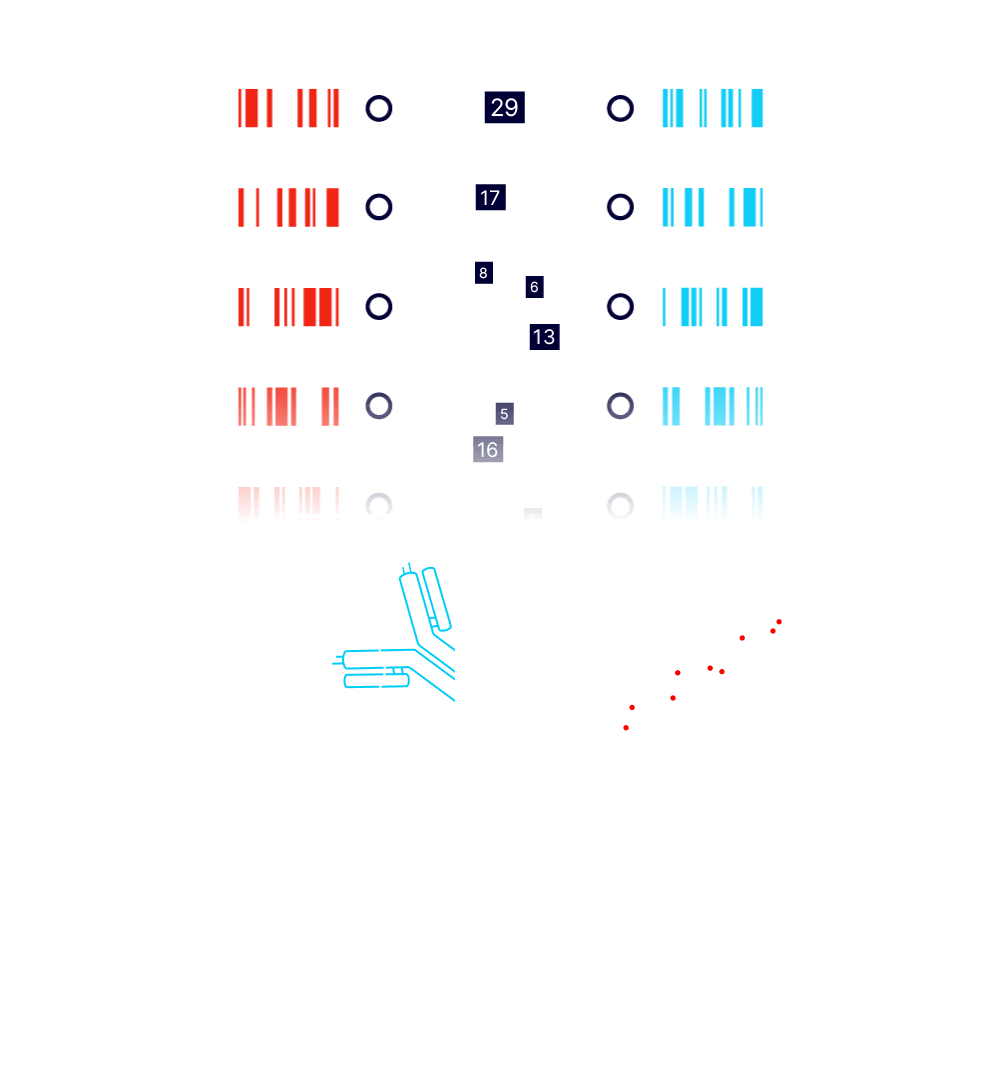
Paired A and Alpha barcodes are read and counted with next generation sequencing. Stronger protein-protein interactions result in a greater frequency of observed barcode pairs. Protein-protein interaction affinities are computed using internal controls and barcode replicates.
![]() AlphaBind
AlphaBindCOMPILE
Data from each new AlphaSeq experiment is added to a growing database of hundreds of millions of protein-protein interaction affinity measurements, including negative data. Control interactions are added to each assay to normalize results between different experiments.![]()
TRAIN
Interaction measurements from the database are used to train machine learning models that relate protein sequence to binding affinity. ML is particularly powerful for teasing apart nonlinear effects from combining mutations, enabling accurate predictions of sequences far from any that have been experimentally tested.![]()
DESIGN
Once trained, ML models are used to predict diverse sequences that have desired binding properties (affinities, specificities, cross-reactivities, epitopes, etc.). Computational screens for expression, stability, solubility, and other properties are then used to enrich designs for those most likely to be developable.![]()
Validate & REFINE
Model predicted protein sequences are synthesized and tested experimentally with AlphaSeq and orthogonal assays. A <6-week AlphaSeq + AlphaBind iteration cycle enables rapid evaluation and improvement of model performance.
For more technical details about our platforms, explore our scientific presentations and publications.
View moreLearn more about how we leverage our technology to advance human health.